FDA Enhances Global Patient and Regulatory Collaborations in Oncology
4.6 (458) · $ 7.99 · In stock
In recognition of World Cancer Day 2024, the FDA and European Medicines Agency will collaborate to spotlight innovative cancer treatment advances for patients.
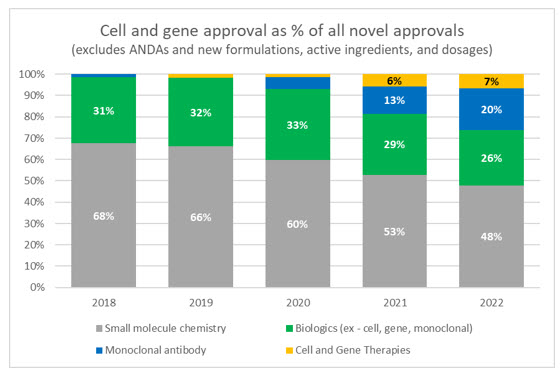
2023's Market Outlook For Cell And Gene Therapies

Jessica Cordes on LinkedIn: #womeninscience #empathy #leadership #collaboration #togetherstronger

FDA Enhances Global Patient and Regulatory Collaborations in Oncology

CML Advocates Network

Rx Rundown: Gilead Sciences, CG Oncology, Genentech and more

Kirsten Boyd Goldberg on LinkedIn: Project Livin' Label

OCE Programs and Projects Overview

FDA's CoGenT Global Revolutionizing Gene Therapy Regulations

FDA posted on LinkedIn

FDA on LinkedIn: #healthequity #patientscience #digitalhealth #clinicaltrial #diagnostics…

Kirsten Boyd Goldberg posted on LinkedIn

Maria Golovina, MS MBA on LinkedIn: FDA Enhances Global Patient and Regulatory Collaborations in Oncology
FDA eyes collaborative review pilot for gene therapies

The Health Equity Update - February 14, 2024 - US FDA