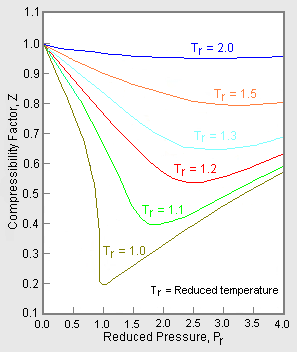
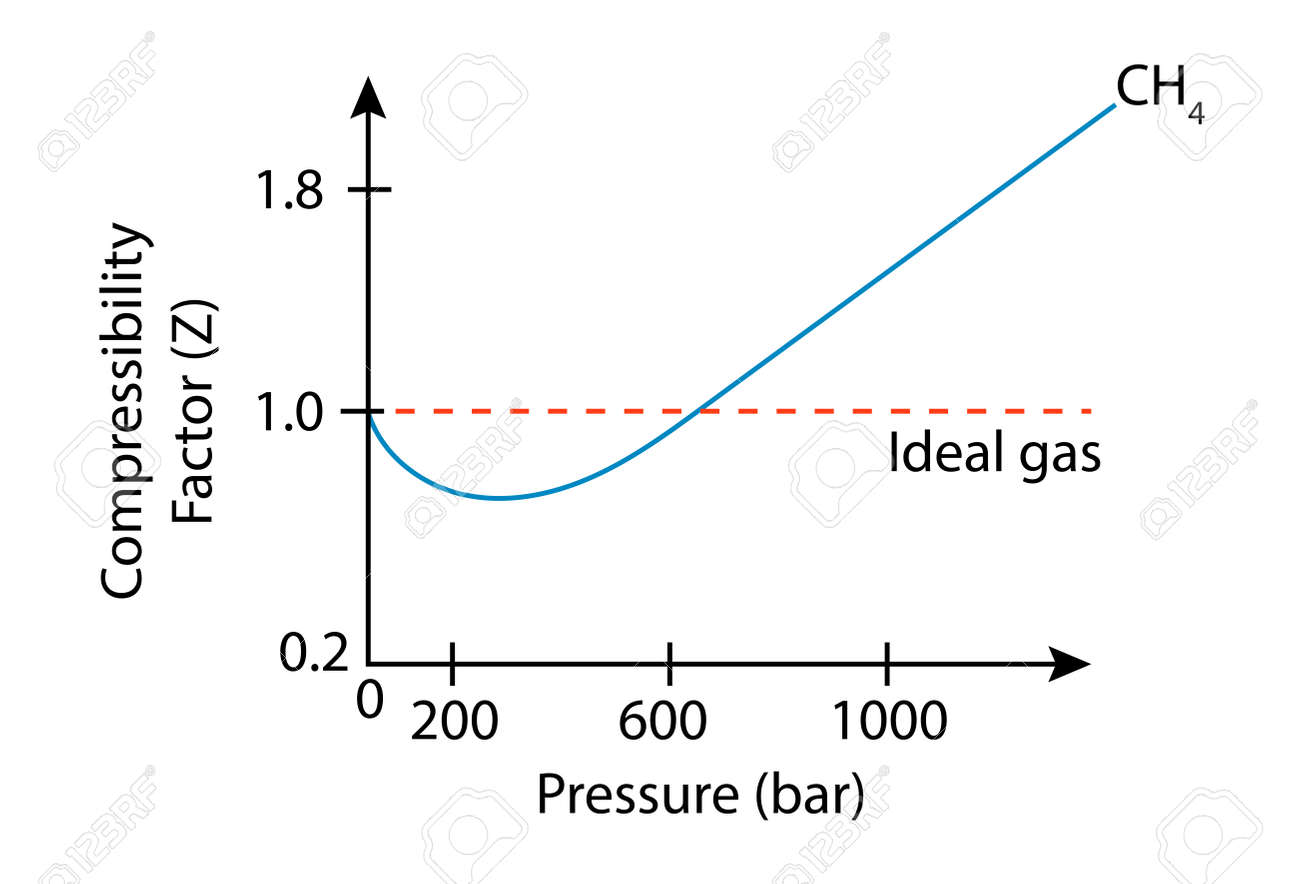
Finding the compressibility factor (Z)
4.8 (583) · $ 15.50 · In stock

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be
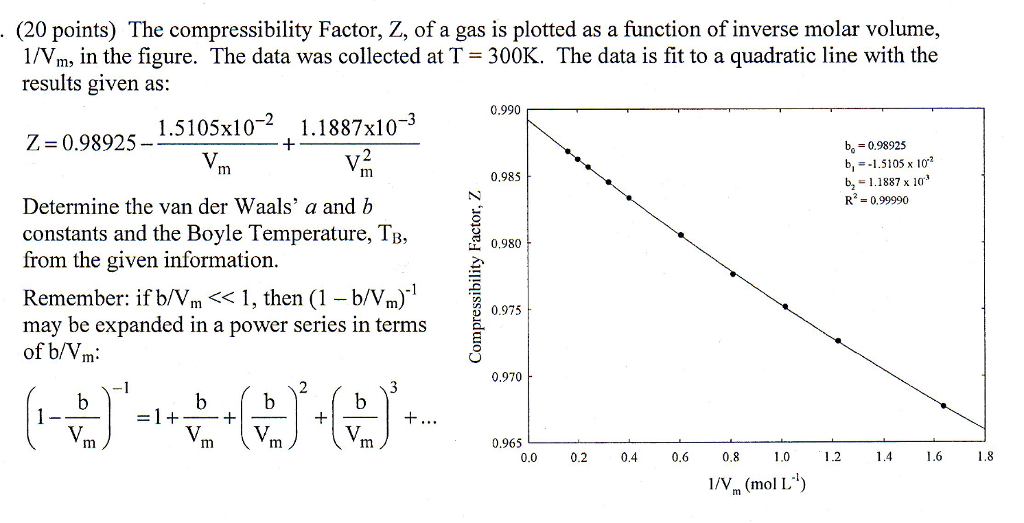
Solved The compressibility Factor, Z, of a gas is plotted as

Plot of experimental measurements of the z-factor

Non-Ideal Gas Behavior Chemistry: Atoms First
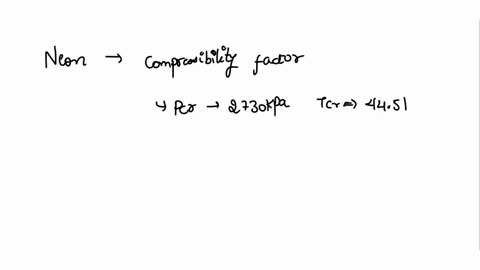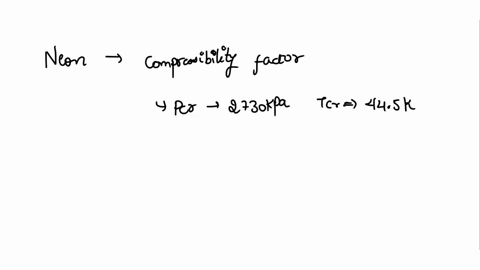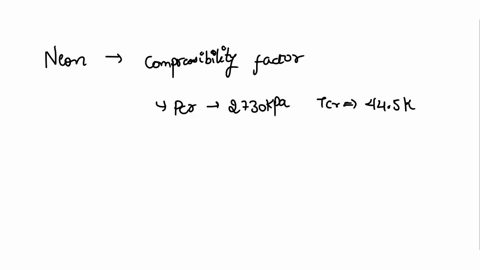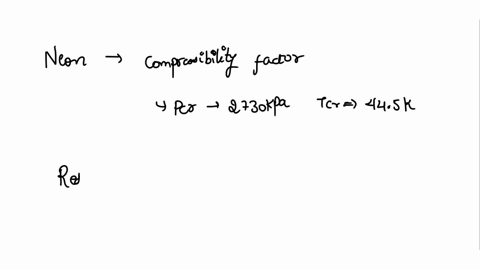
⏩SOLVED:With Tr=0.85 and a quality of 0.6 find the

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

The compressibility factor for nitrogen at `330K` and `800 atm` is `1.90` and `200 atm` is `1.10`. A

⏩SOLVED:With Tr=0.85 and a quality of 0.6 find the
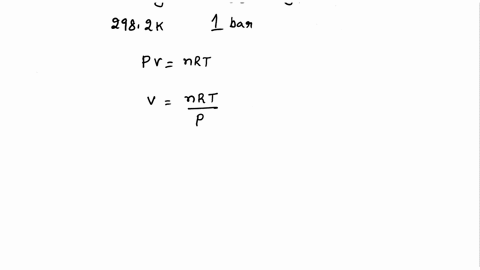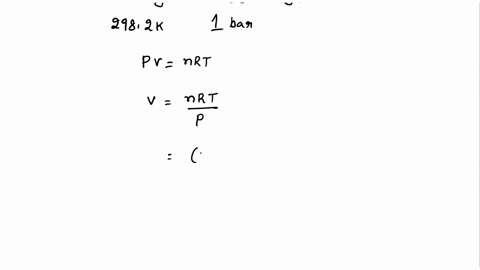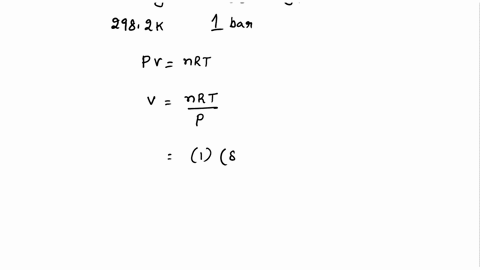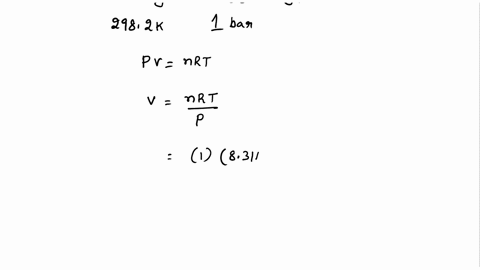
SOLVED: Calculate the molar entropy change when a gas is

Determine Compressibility of Gases
You may also like
Related products
© 2018-2024, banni.id, Inc. or its affiliates