- Home
- compressibility factor equation
- Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
4.6 (621) · $ 13.99 · In stock

Ideal Gases & Real Gases, PDF, Gases

Deviation from ideal gas behaviour

Gas compressibility factor Z: Ideal gas vs Real gas
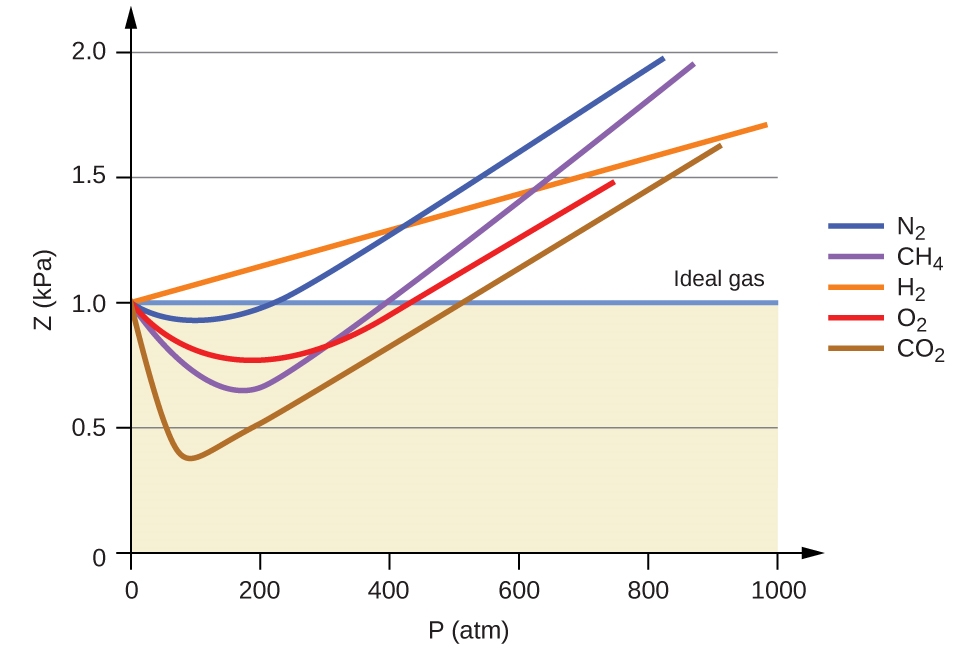
2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
What is the significance of the curve part in Z vs. P graph of

Solved 4. Real gas effects can be expressed as departures

1.reservoir Engineering Notes K PDF, PDF, Gases

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Properties of Gas Manik

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Chemistry!!! Not Mystery : Do Real Gases Behave Ideally?

Compressibility factor `Z=(PV)/(RT)`. Considering ideal gas, real gas, and gases at critical

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics












