In the following compressibility factor Z vs pressure graph at 300
4.9 (252) · $ 19.00 · In stock
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following diagram. The graph is plotted at constant temperature 2 - Sarthaks eConnect

Chemistry Desk: Effect of Pressure
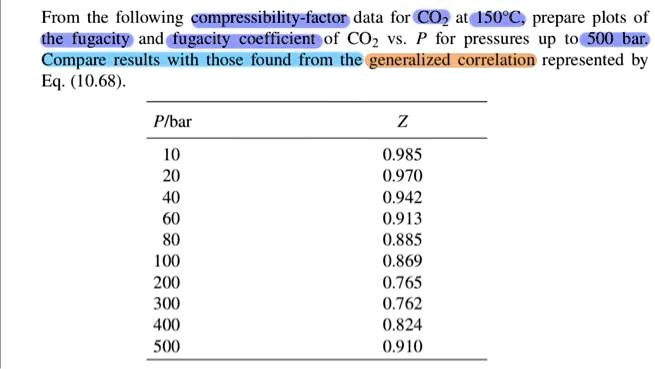
SOLVED: From the following compressibility-factor data for CO at 150°C, prepare plots of the fugacity and fugacity coefficient of CO vs. P for pressures up to 500 bar. Compare the results with
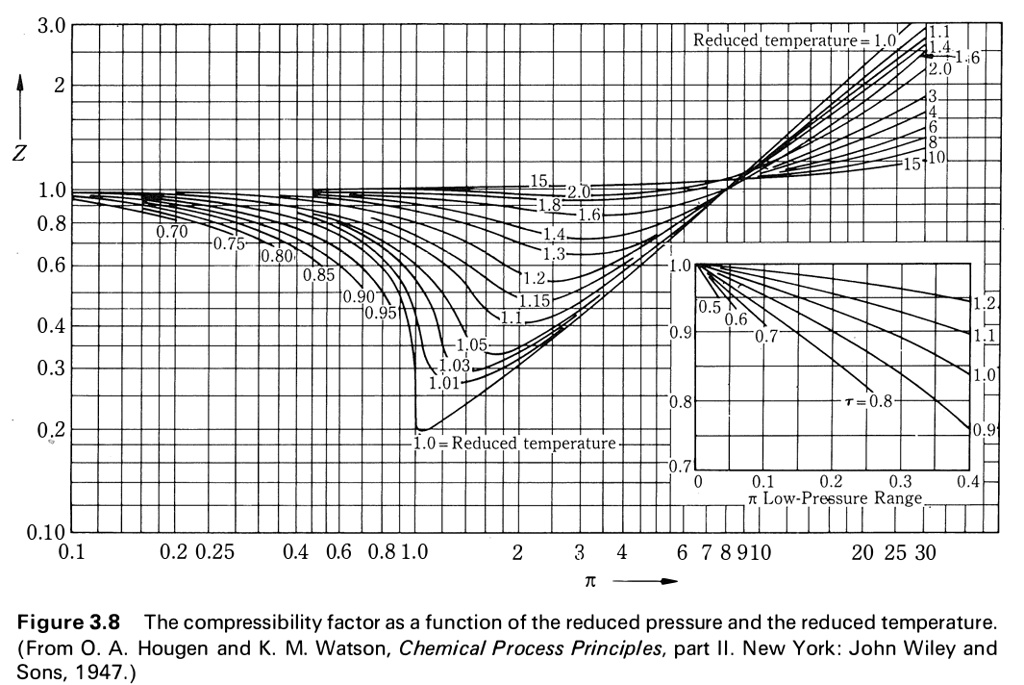
SOLVED: Use the graph of compressibility factors in terms of reduced variables to answer the following about methane (pc = 4.595 MPa, Tc = 190.55 K, and M = 16.04 g/mol): a)

What is the expression of first law of thermodynamics for adiabatic process

Energies, Free Full-Text

Gas Laws / Gases Behaviour, PDF, Gases

The van derWaals constant a for different gases are given below Gas aatmlit2mol2 O2 136 N2 139 CH4 225 NH3 417 The gas that can be most easily liquefied is

Compressibility factor (gases) - Citizendium
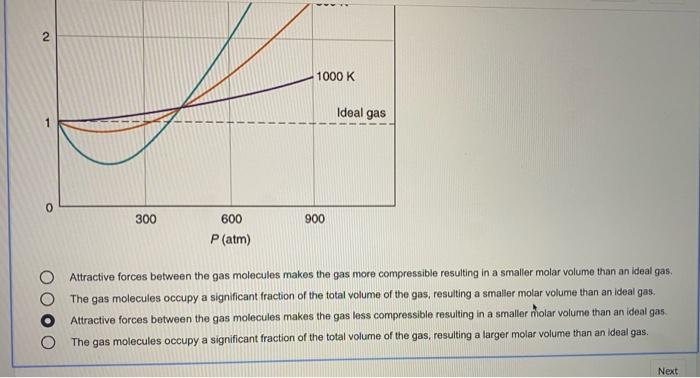
Solved 6 1 point The plot below shows how compressibility

Non-Ideal Gas Behavior Chemistry: Atoms First
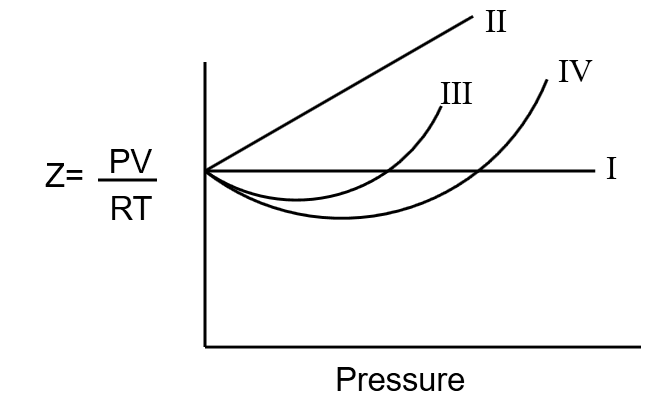
For the nonzero volume of molecules having no forces of attraction the variation of compressibility factor ZPVRTwith pressure is given by graph