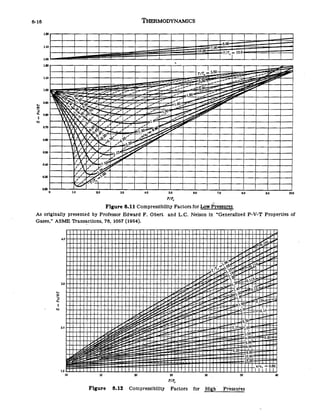
- Home
- compressibility factor z
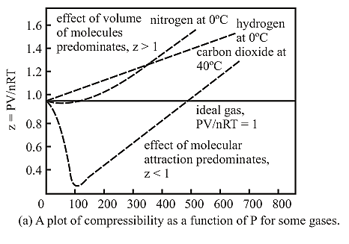
- In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar
In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar
4.8 (162) · $ 12.00 · In stock
Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases

Compressibility Factor Calculator - File Exchange - MATLAB Central
Punjabi] The graph of compressibility factor (Z) vs. P for one mole o
Solved Use the compressibility charts to answer the

ars.els-cdn.com/content/image/3-s2.0-B978012803188

Compressibility Factor Z Important Concepts and Tips for JEE Main
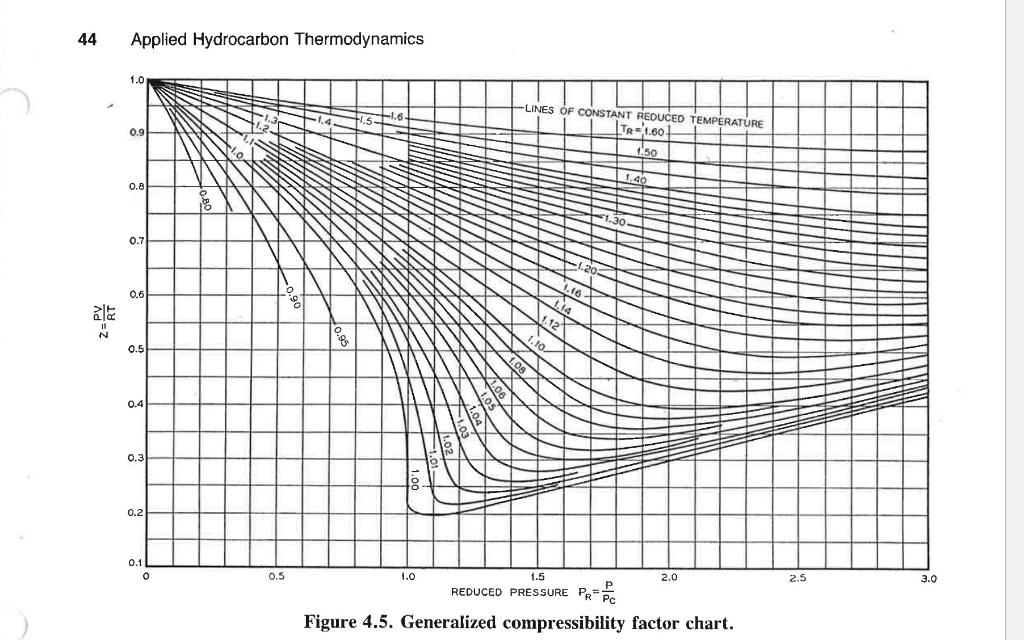
Solved Let us use the generalized compressibility factor
Two flasks A and B have equal volumes A is maintained at 300K and B at 600K While A contain H2 gas B has an equal mass of CH4 gas Assuming ideal
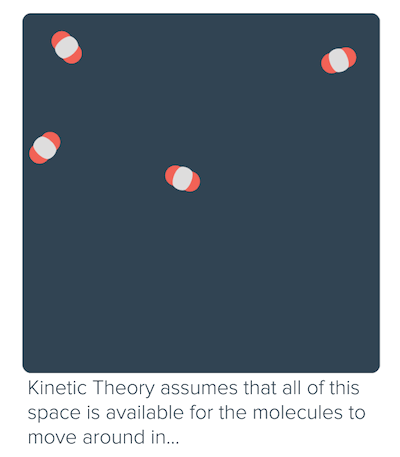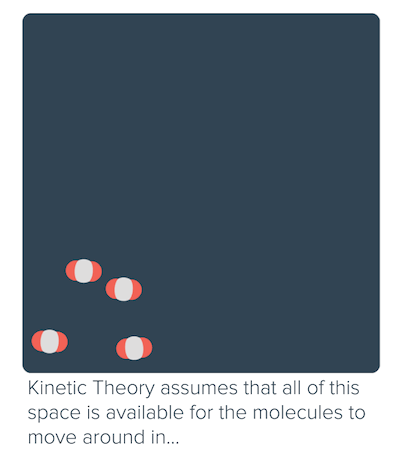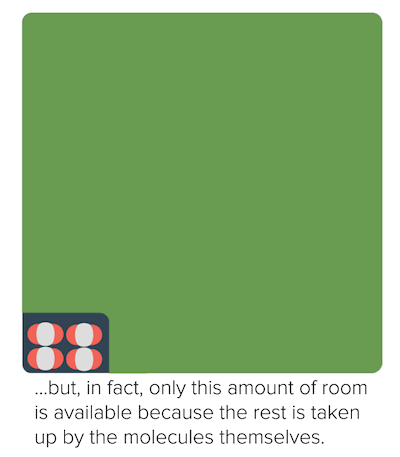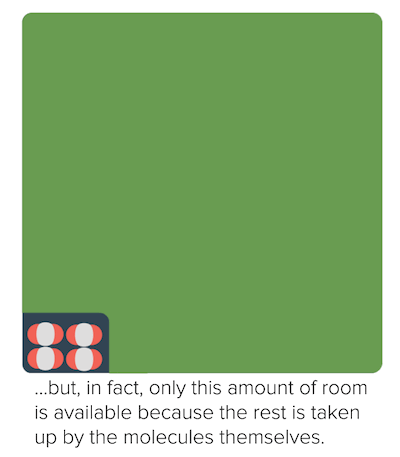
Non-ideal behavior of gases (article)

Part 4. Thermodynamics of Gases - W.H. Freeman

Compressibility factor - Wikipedia

In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure

Compressibility factor (gases) - Citizendium
Real Gases and the Virial Equation