Solved What is the equilibrium constant (Kp) at 45 °C for
4.7 (688) · $ 6.99 · In stock
Answer to Solved What is the equilibrium constant (Kp) at 45 °C for

Consider the reaction: A(g) ⇌ B(g) + C(g) Find the equilibrium co
At 444^° C, the equilibrium constant K for the reaction 2AB gives A2 +B2,The degree of dissociation of AB will be (A) 10
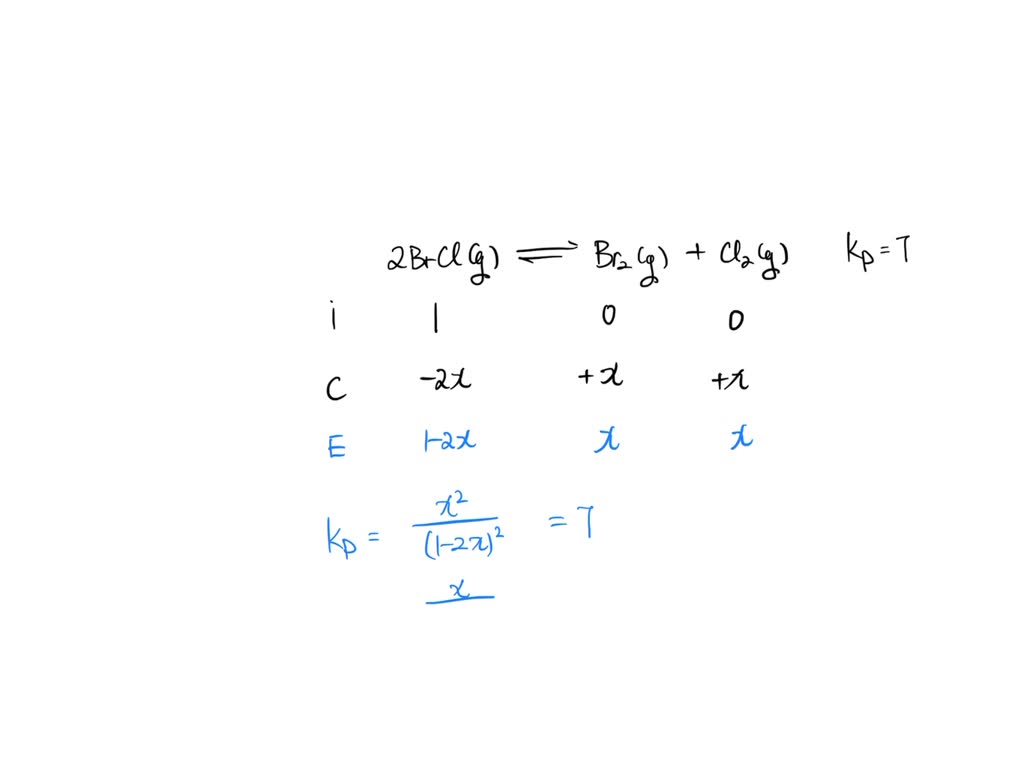
SOLVED: For the equilibrium 2 BrCl (g) Br2 (g) + Cl2 (g), the equilibrium constant Kp is 7.0 at 400 K. If a cylinder is charged with BrCl (g) at an initial

At constant temperature, the equilibrium constant Kp for the decomposition reaction

For the reaction, A(g) + B(g)rightarrow C(g) + D(g), Delta H^o and Delta S^o are, respectively, -29.8 kJ mol^{-1} and -0.100 kJ K^{-1} mo1^{-1} 298 K. The equilibrium constant the reaction 298
search-static.byjusweb.com/question-images/aakash_
How to Calculate the Equilibrium Constant, K
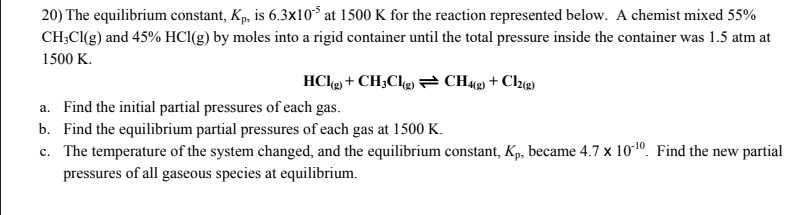
Solved 14) Will decreasing the temperature of the following
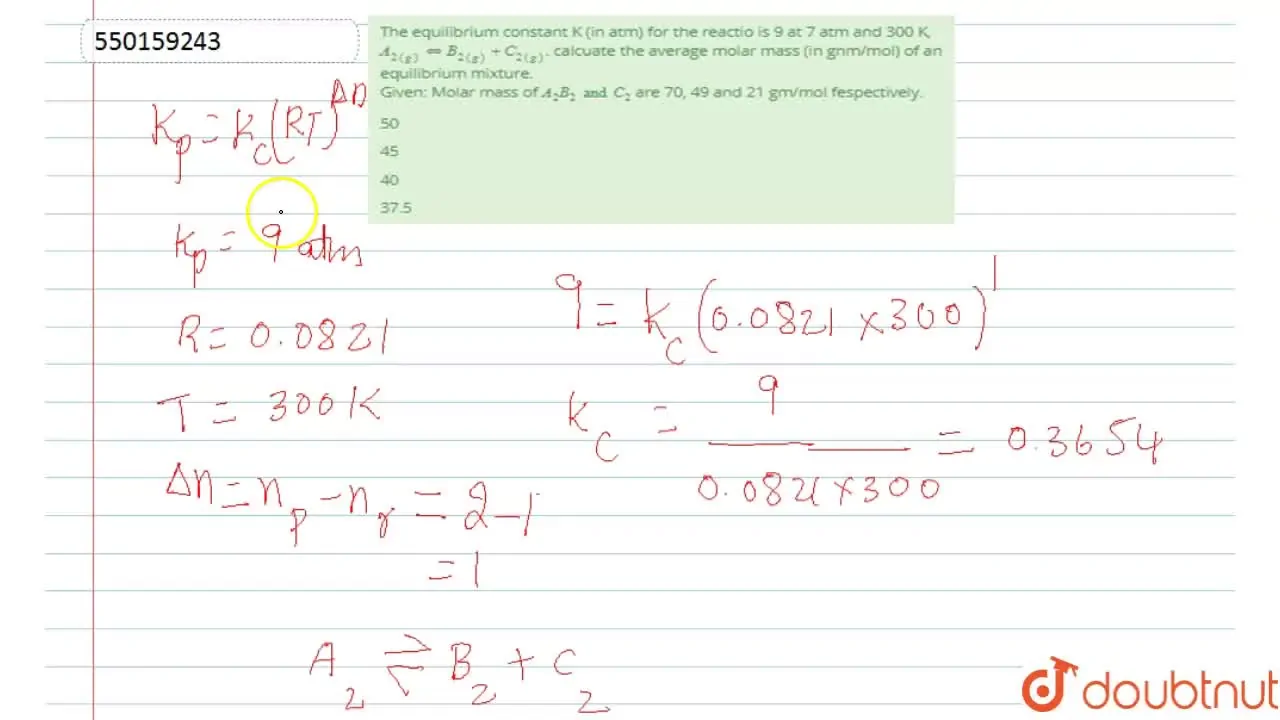
Telugu] The equilibrium constant K (in atm) for the reactio is 9 at 7
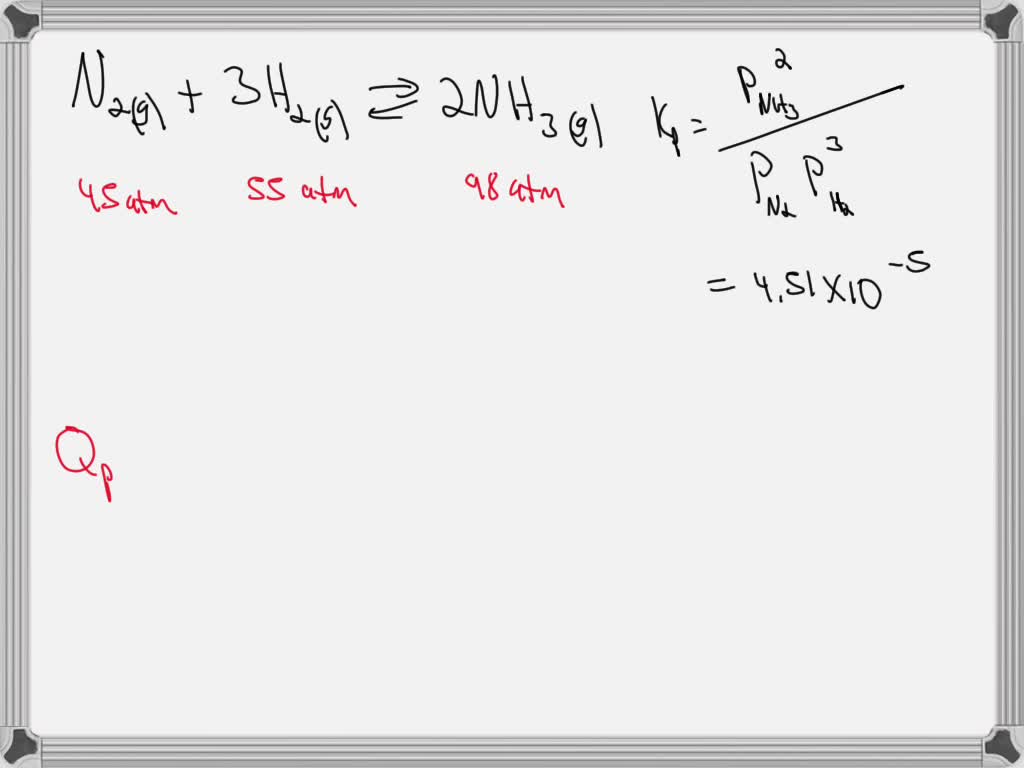
SOLVED: Kp for the equilibrium N2(g) + 3H2(g) ⇌ 2NH3(g) is 4.51 × 10-5 at 450°C. A mixture at this temperature has 98 atm NH3, 45 atm N2, and 55 atm H2.

The equilibrium constant (KP) of the reactions N2O4hArr 2NO2 was found












